
You probably already know what a diamond is: A precious gemstone, the hardest material on earth, a sparkly accessory, a girls best friend, forever - do we need to go on? But did you ever wonder what a diamond is made of, or how it came into existence? If you didn’t, you’re probably wondering right now, so luckily we’re here to give you all the answers!
Once upon a time, in a place far, far away
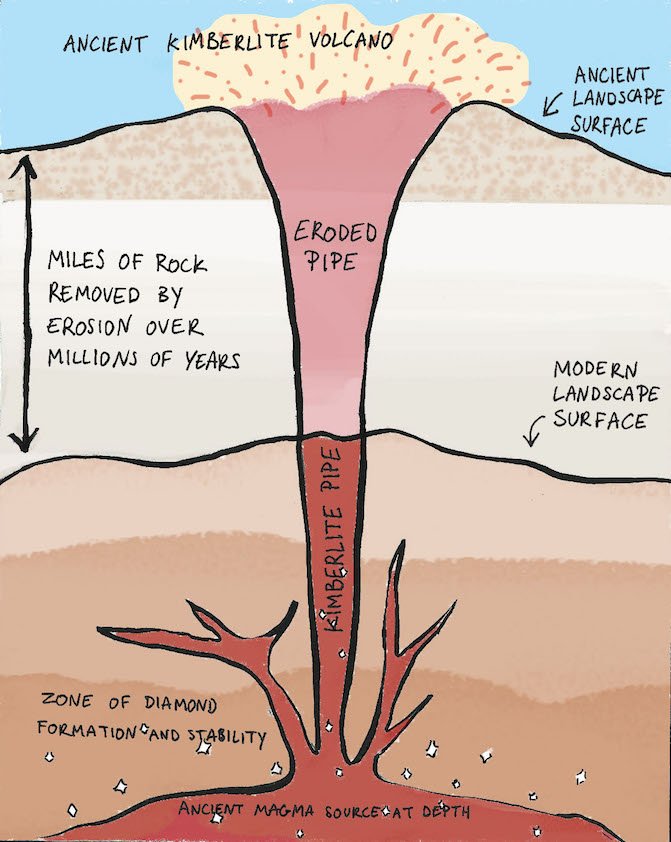
Diamonds exist purely of carbon, the same material a pencil is made of. The way the carbon atoms are arranged gives the stone its unique characteristics. There is one place on earth (or rather in the earth) where the conditions for carbon to turn into diamond are just right, and that is between 140 to 190 kilometres below the earth’s surface. At this depth the temperature is over a 1.000 degrees Celcius, and the pressure is comparable to holding the Eiffel Tower upside down on your finger. This is the perfect environment for a diamond to grow. The formation process takes 1 - 3.3 billion years, which is roughly half the age of the earth! In other words, the diamonds we own today already existed when the earth was still populated by dinosaurs.
Diamonds have been brought close to the earth’s surface millions of years ago, through deep volcanic eruptions which forced the gems up from the depths of the earth mantle. The diamonds are coated by a stone called kimberlite, which prevents the gems from being destroyed in the hot lava. The first diamond-rich volcanic pipe - or ‘kimberlite pipe’ - was discovered in the town of Kimberley in South Africa in the 19th century, hence the name. These pipes are quite rare and can only be found in a few places on earth like Southern Africa, Canada, Russia, Australia and Brazil.
Hardness: 10/10
A diamond has some unique characteristics that give it it’s reputation of being the most valued and precious gemstone we know. It is the hardest material to be found in nature, with a hardness of 10 on Mohs scale. Diamonds also have an extremely high refractive index, which gives the stone its characteristic “fire” and sparkling appearance. The name diamond comes from the ancient Greek word Adámas, meaning ‘unbreakable’, which seems logical but is not completely true. Although a diamond is the hardest material found on earth, it lacks toughness (measuring its resistance against breaking or chipping) and is therefor not unbreakable. But don’t stress - it is still quite tough compared to other gemstones and under normal circumstances breakage is very rare!
Diamonds exist in all kinds of colors. Best-known and most popular is the white, or colorless, diamond but they come in yellow, orange, blue, red, green and everything in between. These colors emerge when the carbon is ‘contaminated’ during the formation of the diamond by other substances such as boron (blue) or nitrogen (yellow), or when the diamond has been exposed to radiation (green) or other circumstances such as lattice defects for brown diamonds or enormous pressure for red and pink diamonds. Contamination with nitrogen is very common, and it’s the reason a lot of white, colorless stones have a slightly yellowish tone. We only speak of a colored diamond, or fancy color, when the yellow is very bright and vivid.

Apart from their hardness and refractive index, diamonds have a very high thermal conductivity. Because of this array of special properties, diamonds are perfect for all kinds of industrial applications. From the scalpel your doctor uses, the moulds that are used to produce electricity cables, to the windows of an airplane, they all contain diamonds in some form. What most people don’t know, is that the majority of diamonds is used for industrial purposes - around 70% of the world production! But don't worry, it still leaves us with enough of the beautiful, gem quality diamonds we love so much. Because let’s face it: in the end, we all love this little stone the most for it’s dazzling, unparalleled appearance!
